
This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products. This is often not the case-people from racial and ethnic minority and other diverse groups are underrepresented in clinical research. Participants in clinical trials should represent the patients that will use the medical products. Others participate in trials because they want to contribute to the advancement of medical knowledge.Įnsuring people from diverse backgrounds join clinical trials is key to advancing health equity. Clinical trials provide another option when standard therapy has failed. Some people participate in clinical trials because none of the standard (approved) treatment options have worked, or they are unable to tolerate certain side effects. Who should consider clinical trials and why?
Clinical trial start up how to#
to learn how to safely use a treatment in a population for which the treatment was not previously tested, such as children.to study different ways to use standard treatments or current, approved treatments so that they will be more effective, easier to use, or decrease certain side effects.to determine whether a new drug or device is safe and effective for people to use.Why are clinical trials done?Ĭlinical trials are conducted for many reasons:
Clinical trial start up trial#
Learn more about the basics of clinical trial participation, read first hand experiences from actual clinical trial volunteers, and see explanations from researchers at the NIH Clinical Research Trials and You Web site. These rules make sure that those who agree to participate are treated as safely as possible. Similarly, researchers, doctors, and other health professionals who manage the clinical trials must follow strict rules set by the FDA.
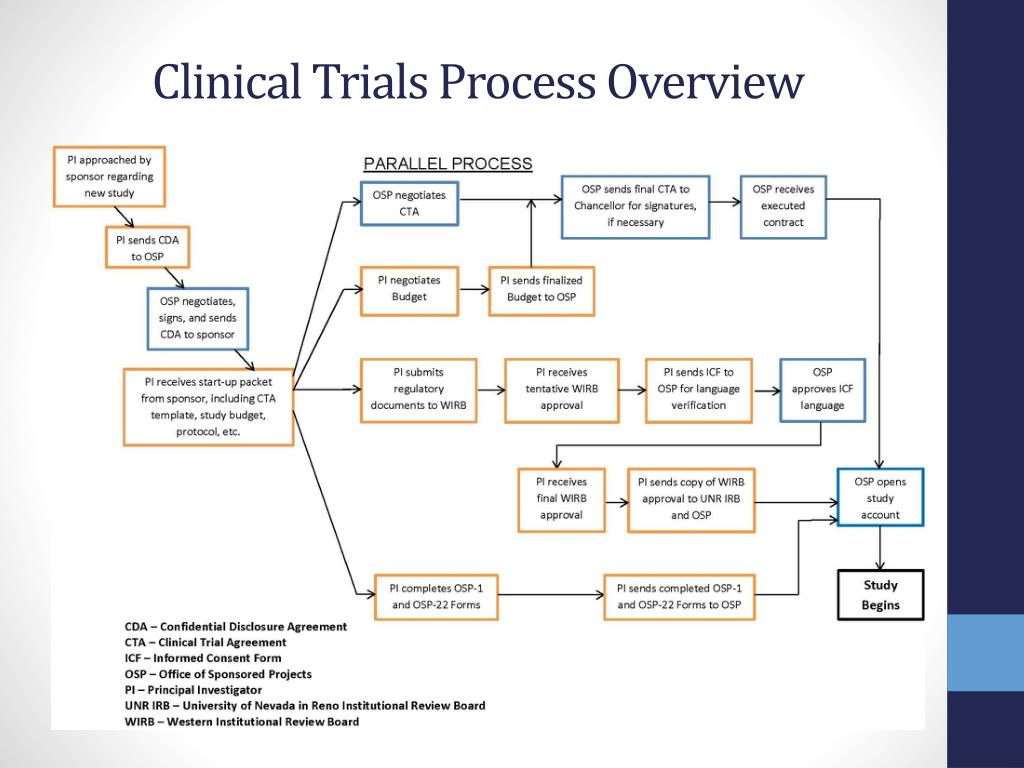
Volunteers who participate in the study must agree to the rules and terms outlined in the protocol. what the researchers hope to learn from the study.the types of patients who may enter the study.When carefully conducted, they are the safest and fastest way to find new treatments and ways to improve health.Ĭlinical trials are conducted according to a plan, called a protocol, which describes: Clinical trials are research studies in which people volunteer to help find answers to specific health questions.


 0 kommentar(er)
0 kommentar(er)
